It’s a little after 8 a.m. on a November day in 2014 when they drop anchor. The waters of the Red Sea an hour off the coast of Yanbu, Saudi Arabia, are amazingly clear. The pale, sandy bottom located 120 feet below practically glows. U-M professor David Sherman adjusts his scuba mask. Soon, he and his colleagues slip into the 82 degree water, making their way toward an old shipwreck nestled among coral and sponges.
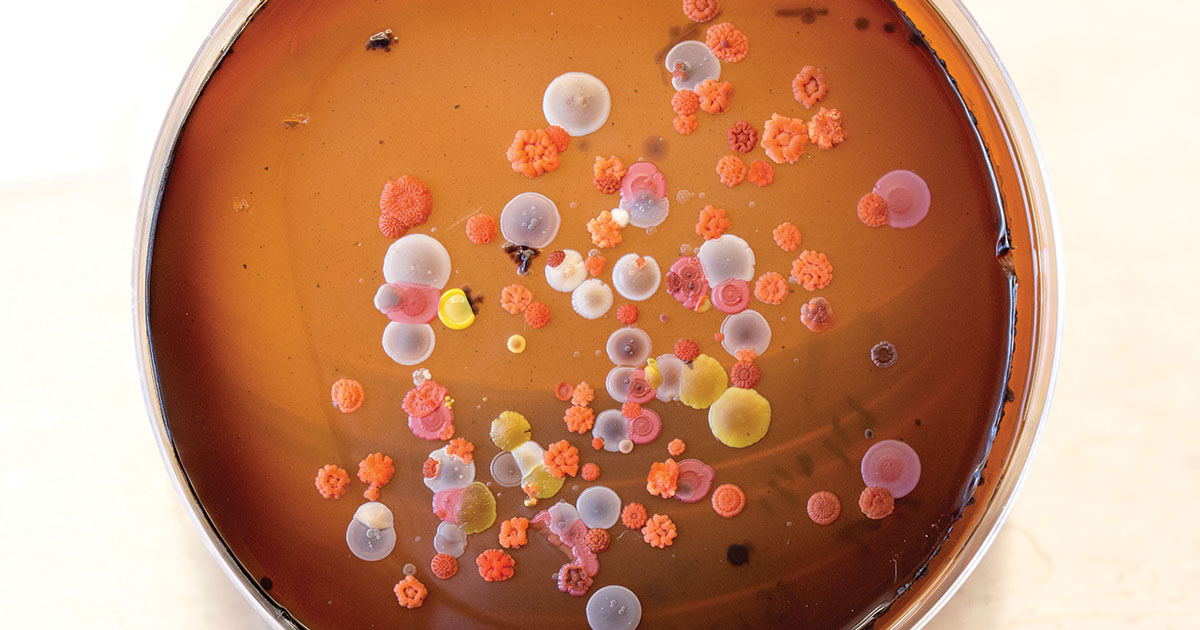
The purpose of the trip is to collect sediment samples from this remote stretch of seafloor. Sherman isn’t interested in the sand and muck, but rather in the bacteria and fungi that live there. Back in the lab, these microorganisms will be cultured and tested to see if the chemicals they produce can kill dangerous pathogens or cancer cells.
“If the answer is yes, we work to pinpoint the bioactive molecule that is responsible,” says Sherman. “Hopefully, it’s something new, something we’ve never seen before. This gives us the starting point for developing new antibiotics and other drugs.”
The Saudi Arabia expedition is just one of many Sherman has undertaken over the course of his decades-long career. Collection trips have also taken him to the sweltering deserts of Israel, the crags of the Himalayas, the rainforests of Central America, and underwater sites off Costa Rica and Papua New Guinea. His collaborators include scientists in more than 30 countries and extend to every continent, including Antarctica.
In the samples Sherman and his team have brought back, they’ve found new antibiotic agents that are effective against some of the most recalcitrant infections, identified a promising new approach for defeating HIV, and isolated a microbe that makes a naturally occurring cancer drug—to give just a few examples.
“When people think of microbes, they think of the bad ones, the ones that cause disease,” says Sherman, a second-generation chemist whose passion for discovery is infectious. “Nobody, except for microbiologists, pays much attention to what I like to call ‘microbes of merit.’ They do their work silently—in the soils, in the ocean—and only by going out to these areas to collect samples can we really get a sense of what’s there.”
A single gram of soil—which weighs about as much as a large paper clip—can be home to tens of thousands of different types of bacteria, fungi, and algae. And Sherman knows that any one of them, over its eons-long battle to survive, may have naturally evolved complex chemical defense mechanisms that could lead to the next lifesaving medicine.
Scientists call these beneficial microbe-made molecules “natural products.”
“Humans have been harnessing the beneficial properties of nature’s chemistry for millennia,” Sherman says. “The more remote and undisturbed the location, the better the chances of finding microorganisms that haven’t been studied before.”
The most famous example of a natural-product drug is penicillin, an antibiotic derived from the fungus “Penicillium notatum.” Since its introduction in the early 1940s, it’s been credited with saving at least 200 million lives.
However, many dangerous microbes are evolving resistance to our most widely used antibiotics. The World Health Organization has called antibiotic resistance an “increasingly serious threat to global public health that requires action across all government sectors and society.” Without effective antibiotics, not only would staph infections and tuberculosis be untreatable, but surgery and cancer chemotherapy also would become much riskier. That’s why scientists like Sherman have been working to develop new antibiotics to stay a step ahead of the problem.
“Along with developing new drugs, controlling the spread of multiple-drug-resistant forms of pathogens is something we need to keep getting better at, including tracking infections and making sure they aren’t able to establish themselves inside hospitals, where they’re very difficult to get rid of,” Sherman says.
He and his collaborators at the National Biodiversity Institute in Costa Rica discovered a new antibiotic on one expedition that, in the lab, has proven effective against anthrax—a potential biological weapon—and against a tenacious type of staph infection known as “methicillin-resistant Staphylococcus aureus,” or MRSA. Moreover, the compound they discovered attacks these infections in an unusual way that limits a bacteria’s harmfulness by blocking its ability to collect the iron it needs to survive and grow.
Many antibiotics are effective against only one type of bacteria—called “Gram-negative” or “Gram-positive,” depending on the structure of their cell wall—but this new class of compounds produced by the marine microorganisms is effective against both.
“It’s a rare example of a broad-spectrum antibiotic,” Sherman says. The team named the new antibiotics “baulamycins” to commemorate Costa Rica’s Las Baulas National Marine Park, where they were discovered.
Sherman’s lab also teamed up with researchers at the U-M School of Public Health on an antibiotic compound that shows promise against an infection called “Acinetobacter baumannii,” which gathers in sticky clusters, or biofilms. Biofilms are difficult for antibiotics to penetrate and kill. In hospitals, they cling to medical devices, prosthetic implants, and other surfaces, making them resistant to sterilization with traditional methods.
Dubbed “cahuitamycins,” the compounds were also developed from a sample collected in Costa Rica’s Cahuita Marine National Park, and efforts to develop them continue. Still, even promising scientific leads like these are extremely challenging to translate from the laboratory bench into an FDA-approved medicine. Drug development is quite complex and expensive, and can take a decade or more. Both of these projects are numerous steps—and at least several years—away from potential clinical use.
Over the past decade, U-M professor Kathleen Collins and her team have shown how Nef, a protein made by HIV, is a key player in the virus’s ability to hide from the defenders in the body’s immune system. Treatments have vastly improved in recent years. But 37 million people worldwide live with HIV, and each year AIDS kills more than 1 million people.
Collins figured that if she could find a drug that could stop Nef, she and her team could remove HIV’s camouflage and allow the body to fight it off as it would a cold virus. They tested a huge library of potential drugs, but none worked.
Then, the U-M Center for Chemical Genomics, based at the U-M Life Sciences Institute—where Sherman’s lab is located—helped Collins test more than 26,000 microorganism-derived extracts from a library built primarily from Sherman’s expeditions. After narrowing the results down to 11 promising compounds, they zeroed in on the three with the most potential to inhibit Nef. All were made by marine “actinomycetes” bacteria. Now, Collins and Sherman are collaborating to develop the results further.
“We’re still at the early stages of this project,” Collins says. “No one has developed a Nef inhibitor with the level of activity that we’re seeing. If this works, we could essentially harness a person’s own immune system to wipe HIV out of the body.”
The work has received funding from the National Institutes of Health, U-M’s A. Alfred Taubman Research Institute, and the Life Sciences Institute’s philanthropy-funded Innovation Partnership program. But the process of developing a tiny bacterial metabolite into an approved drug will require additional funding—and if research continues to be successful, the goal would be to partner with a pharmaceutical company to complete development and testing.
“We’re up against a formidable foe,” Collins adds. “AIDS isn’t going to go away until we have a true vaccine or cure.”
Because each bacterium produces only a tiny amount of a medicinal compound, one of the biggest challenges for the Nef project—and for Sherman’s other work—is being able to produce substantial quantities of the medicinal molecules in a cost-efficient, sustainable way.
In addition, some bacteria are symbionts that live inside a host animal, relying on them for almost everything they need to stay alive, making it extremely difficult to grow them outside that host.
This was the case with a bacterium that Sherman’s group isolated from a sea squirt known as a mangrove tunicate. These brightly colored invertebrates were the longtime source of the cancer drug ET-743, or trabectedin. But it was Sherman’s lab that pinpointed the bacteria within the creatures that actually produce the cancer-fighting molecule—an important step toward being able to produce the drug more sustainably.
“You can imagine what a huge difference it would make if we could culture the bacteria that makes the drug directly,” says Sherman. “It also opens up opportunities to genetically modify the organism to produce new, related compounds.”
Sherman’s expeditions to far-flung places have a symbiotic purpose of their own—something one might call “scientific diplomacy.” His group is forming its own complex web of partnerships and collaborations, building relationships, and sharing scientific knowledge.
“When we go to these countries, we bring capacity-building,” says Sherman. “We bring the expertise to help them develop a sustainable research program in natural product discovery, and that leads to the potential for drug discovery. And the potential for drug discovery is of high interest to most scientists who are concerned about improving human health.”
The benefits of this type of open, collaborative science can extend far beyond the specific researchers or labs involved. Sherman’s collaborations, for example, often lead to the characterization of new natural products, which researchers across academia and industry can analyze for their disease-fighting capabilities.
And beyond benefiting specific institutions, data published in a recent Nature commentary titled “Open countries have strong science” showed that international collaborations can benefit an entire scientific field. The analysis of publishing and citation data revealed a strong correlation between a nation’s “openness”—the number of studies it publishes with authors from other nations as well as the ability of its researchers to relocate—and its scientific impact.
The authors of the commentary emphasize that nations wanting to maximize their scientific impact would benefit from increased international collaboration, and they recommend that funding agencies “move away from policies that fund only national researchers” and instead link domestic scientists to the best science, “wherever it is.”
Last year, Sherman and one of his graduate students, who is also a master diver, participated in a scuba expedition in Cuba that brought international scientists together around improving marine conservation practices and policy.
“It’s a wonderful thing,” Sherman says of the trip, “to be able to put politics aside and come together with researchers from around the world to work on important scientific questions and problems that affect us all.”
Ian Demsky has been a writer and editor at U-M since 2010. He previously worked as a newspaper reporter in Nashville, Tennessee; Portland, Oregon; and Tacoma, Washington.